2.1. Cyclic Voltammetry Analysis of O2 in the Presence of 5-ASA and 4-ASA
O
2•− elimination by 5-ASA, 4-ASA, and several related compounds (
Figure 1) was investigated on the basis of electrochemical measurements in DMF.
Figure 2 shows the cyclic voltammetry (CV) results for saturated O
2 (4.8 × 10
−3 mol dm
−3) in the presence of the aminophenols (Ph(NH
2)OH), 5-ASA,
p-AP, 4-ASA, and
m-AP; and the dimethylaminophenols,
p-DAP and
m-DAP. The voltammograms obtained in the presence of
p-AP and
p-DAP were reported in our previous paper, and are presented here for comparison [
12]. In aprotic and unbuffered solvents such as DMF, O
2 shows quasi-reversible redox (Equation (1)), corresponding to the generation of O
2•− in the initial cathodic scan and reoxidation to O
2 in the returned anodic scan (solid lines,
Figure 2a–f) [
9,
10,
11,
12,
13,
15]. The reversible CV behaviors investigated here are all modified to irreversible CV behaviors by the presence of all the compounds (a–f) in a concentration-dependent manner (0 to 5.0 × 10
−3 mol dm
−3), similar to that observed for typical phenolic compounds (
Supplementary Information, Figure S1). This is supported by the fact that the CV traces in the absence of O
2 (removed by bubbled N
2) show no peaks over the potential range investigated (data not shown). Thus, the loss of reversibility in the O
2/O
2•− couple is due to the initial PT from the compounds forming HO
2• (Equation (2)).
With the generation of HO
2•, the bielectronic voltammograms observed are derived from the reduction of HO
2• (Equation (3)) as shown in
Figure 2a,c–f. Conversely, in the presence of
p-AP, the CV traces do not show bielectronic characteristics owing to the elimination of HO
2• by the subsequent ET (Equation (4)) from its phenolic anion (
p-Ph(NH
2)O
−). In our previous study, the ET was experimentally observable by electron spin resonance (ESR) measurements owing to the radical product of the ET coupled with the second PT (Equation (5)), in which the
para-amino group donates another proton [
12]. However, the CV results obtained in the presence of 4-ASA and
m-AP (
Figure 2d,e) present bielectronic curves, which suggests that HO
2• (O
2•−) is not eliminated through ET from the
m-AP moiety.
These results confirm that the quinone–hydroquinone π-conjugated redox system [
10,
11] characteristic of the
p-AP moiety plays an important role in PCET involving two PTs and one ET. Thus, for successful O
2•− elimination through PCET, two structural characteristics are required; (1) an amino proton for the second PT, and (2)
para-substitution (i.e., the
p-AP moiety) for π-conjugation.
Considering these results, we rationalized that O
2•− formation after the primary electrode process associated with PT from the phenolic hydroxyl group leads to the irreversible overall reduction of O
2 to H
2O
2, which is driven by the exergonic reduction of the resulting HO
2•/HO
2−. Therefore, the CV traces for O
2/O
2•− in the presence of phenolic compounds are divided into two typical curves: type A, an irreversible two-electron process observed in electro–chemical–electro (ECE) reactions (Equations (1)–(3)), and type B, an irreversible one-electron process (Equations (1), (2), (4) and (5)) leading to O
2•− elimination.
Figure 3 shows the plausible electrochemical mechanisms for O
2/O
2•− in the presence of
p-AP and 5-ASA, summarizing Equations (1)–(5).
In this scenario, the CV results recorded in the presence
p-AP demonstrate type B (elimination of O
2•−). Conversely, the others involving both 5-ASA and 4-ASA demonstrate type A (O
2•− is not eliminated) showing the appearance of a cathodic current ascribed to HO
2•. In
Figure 2a,d, the reduction current for HO
2•/HO
2− appears as a clear prepeak in the positive side of the O
2/O
2•− peak due to the acidity of 5-ASA and 4-ASA derived from 1-carboxyl group. The voltammetric behavior for O
2 in the presence of 5-ASA (and 4-ASA) is different from that of O
2 in the presence of
p-AP, despite the fact that 5-ASA contains a
p-AP moiety in its structure. These CV results indicate that the 1-carboxyl group of 5-ASA suppresses ET via the PCET mechanism from the
p-AP moiety and that single PT occurs from the carboxyl group instead. Furthermore, HO
2• formed by PT from the carboxyl group does not oxidize the
p-AP moiety of the generated 5-ASA anion. Thus, this confirms that the ET is coupled with PT from a phenolic hydroxyl group or amino group, but not from the 1-carboxyl group. Therefore, the 1-carboxyl proton of 5-ASA inhibits PT from the phenolic hydroxyl group or amino group, resulting in the suppression of O
2•− elimination through the net PCET.
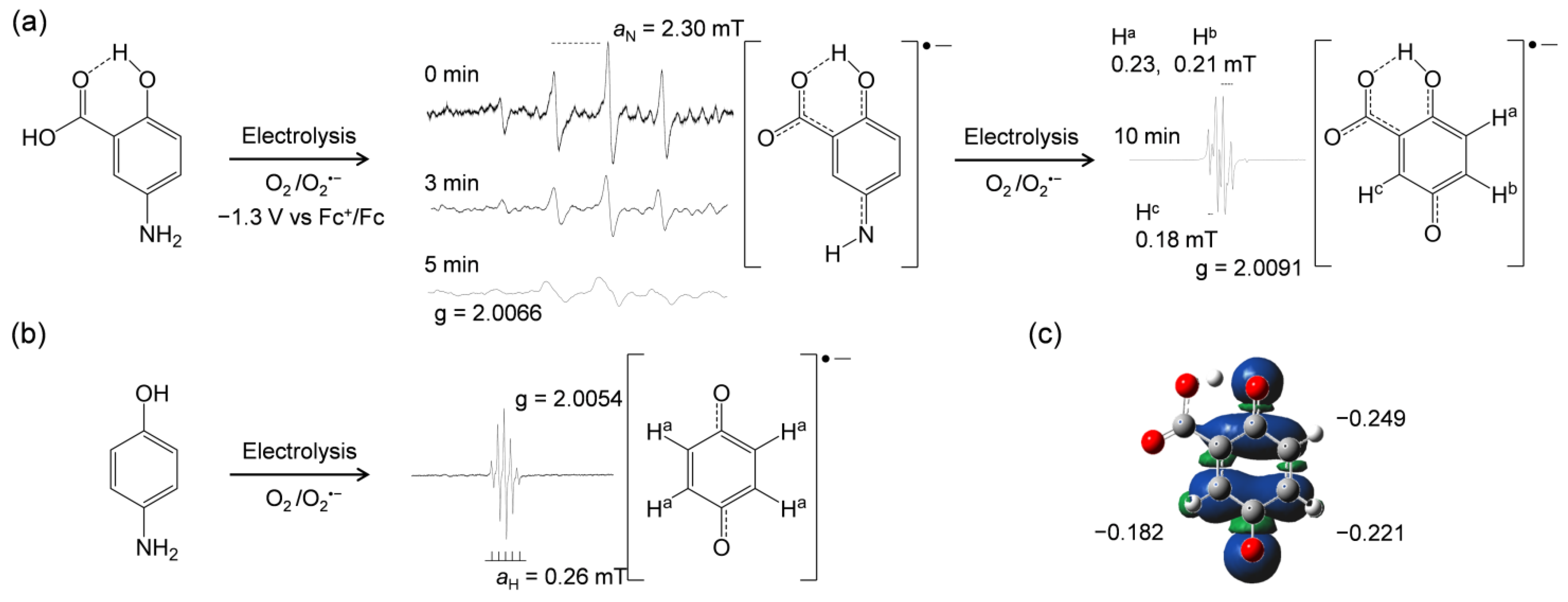
2.2. In Situ Electrolytic ESR Analyis of O2/O2•− in the Presence of 5-ASA and the Related Compounds
The ET from 5-ASA and the related compounds (
Figure 1) to O
2•− and/or HO
2• has been inferred from in situ electrolytic ESR measurements. The ESR spectra of O
2 solutions containing the compounds were measured using controlled-potential electrolysis at an applied potential of −1.3 V corresponding to O
2 reduction (Equation (1)). ESR spectra were only obtained for the CV solutions containing 5-ASA or
p-AP (
Figure 4). As reported previously, the spectrum obtained for
p-AP was assigned to 1,4-benzoquinone radicals based on the comparison of the experimental spectrum with the simulated hyperfine coupling constants (HFCCs) for hydrogen (
aH) [
12]. The ESR result shown in
Figure 4b indicates that not only the ET but also an exchange of a nitrogen atom with an oxygen atom occurs in the
p-AP moiety. However, the mechanism of this exchange is as yet unknown. Notably, ESR spectra were also obtained for 5-ASA (
Figure 4a), despite no ET being indicated in the CV results (
Figure 2a) at the timescale used. A spectrum with a large splitting at 2.30 mT, which may be derived from the amino nitrogen (
aN), was initially observed (0 min; scanning initiated at the same time as voltage applied) upon ESR scanning for 3 min. However, this soon disappeared under the applied potential (see results obtained at 3 and 5 min) [
16]. After 10 min of applied potential, another spectrum was presented, showing small splittings at 0.23, 0.21, and 0.18 mT.
Considering the ESR results for
p-AP (
Figure 4b), the
p-AP moiety of 5-ASA is expected to react with O
2•− through PCET, because the initially observed ESR spectrum seems to be derived from a 1,4-quinone-imine-related radical. This result is significant in that it confirms the O
2•− elimination ability of 5-ASA, which is not observed for 4-ASA. Furthermore, the changes in the ESR spectrum suggest that the nitrogen atom of the 5-amino group exchanges with an oxygen atom by way of a 1,4-quinone imine radical. After this change, the ESR spectrum of the product can be assigned to the radical anion of 1,4-benzoquinone-2-carboxylic acid [
17], as supported by the simulated results.
Next, the spin distribution on the radical product was calculated by DFT at the DFT-B3LYP/DMF/6-311+G(d,p) level, and the results are shown in
Figure 4c. Furthermore, the charge numbers corresponding to the spin electron were obtained by the natural bond orbital (NBO) analysis (
Supplementary Information, Table S1). The calculated charges on the 1-, 2-, and 5-position carbons of the quinone radical moiety denoted in
Figure 4c are in good correlation with the HFCCs observed in the experimental ESR results for the product radical. Based on these results, the HFCCs were assigned as H
a: 0.23, H
b: 0.21, and H
c: 0.18 mT, accurately identifying the product radical structure.
As shown above, the 1-carboxyl group suppresses PCET at the CV timescale by donating the carboxyl proton to O2•−, forming the deprotonated 5-ASA anion. However, this deprotonation enables subsequent PCET from the p-AP moiety to another O2•−. Therefore, it is reasonable to assume that the reaction product is a 1-carboxyl benzoquinone radical, formed through sequential 1:2 reactions, the initial PT of the 1-carboxyl proton to O2•−, and the subsequent PCET between another O2•− molecule and the p-AP moiety followed by exchange of the nitrogen atom with an oxygen atom. These experimental results demonstrate that 5-ASA can eliminate O2•− through PCET in basic DMF solutions.
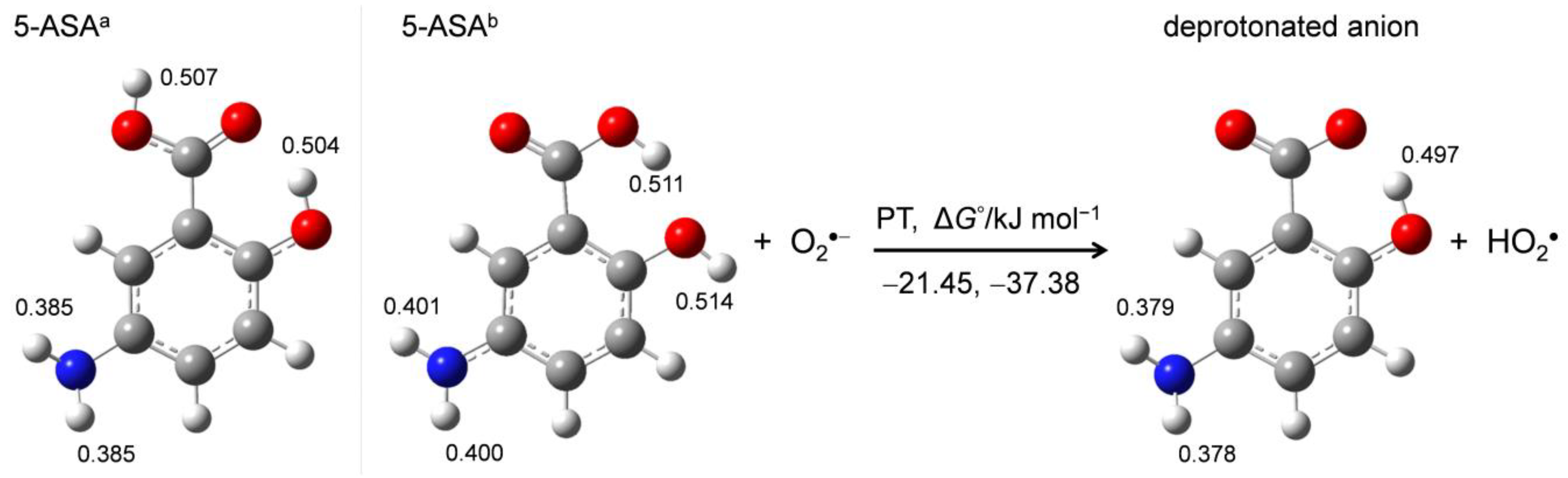
2.3. Stable-Structure Optimization for 5-ASA and Its Anion by DFT Calculation
DFT calculations were performed to aid the mechanistic analysis of O
2•− elimination by 5-ASA. First, in order to obtain the structures of the stable conformer of 5-ASA and its corresponding anion, energy scanning for the dihedral angle around the 1-carboxyl group was performed.
Figure 5 shows the optimized structures of two 5-ASA conformers (5-ASA
a, 5-ASA
b) and the deprotonated anion upon initial PT. Calculated standard Gibbs free energy changes (Δ
G°/kJ mol
−1, 298.15 K) for the PT and distributed charges on the acidic protons obtained by NBO analysis (
Supplementary Information, Table S2) are denoted.
5-ASA has two planar conformers that differ in the orientation of the 1-carboxyl group, forming an intramolecular hydrogen bond (HB) between adjacent 2-hydroxyl groups. Comparison of the charge distribution on the acidic protons shows that the 1-carboxyl and 2-hydroxyl protons are more positively charged than the 5-amino protons, suggesting that the 1-carboxyl or 2-hydroxyl proton is transferred in the initial acid–base reaction to an alkali O
2•−. The Δ
G° values show that 5-ASA
a is 15.93 kJ mol
−1 more stable than 5-ASA
b in DMF, though the PT from 5-ASA
a (−21.45) and 5-ASA
b (−37.38) are exergonic reactions. For either conformer, the proton that is not involved in the intramolecular HB that is transferred to O
2•−, forming a conformer of the corresponding anion that is stabilized by the intramolecular HB [
18], as shown in
Figure 5. These calculation results demonstrate that the initial PT occurs from the 1-carboxyl or 2 hydroxyl group forming the stable anion, which involves the
p-AP moiety having an intramolecular HB.
2.4. Change in HOMO–LUMO Energies upon PCET Between O2•− and 5-ASA
For a detailed analysis of the PCET involved in O
2•− elimination by 5-ASA, HOMO–LUMO changes upon PCET between O
2•− and 5-ASA were calculated by the DFT method (
Figure 6). After the initial PT, some reactant species, i.e., 5-ASA, the deprotonated (−H
+) 5-ASA anion, the dianion, O
2•−, and HO
2• coexist in the solution. The SOMO energy (Hartree) for HO
2• (−0.31659) is much lower than HOMO energies of 5-ASA, the anion, and the dianion. Thus, the electron acceptor will be HO
2•, not O
2•−. Considering that CV revealed that HO
2• is not eliminated by the 5-ASA anion (
Figure 2a), the electron donor will be the dianion, for which the downhill energy relationship is indicated by the bold red line in
Figure 6. Thus, this change in HOMO–LUMO energies upon PT between the 5-ASA anion and O
2•− forming the dianion and HO
2• is reasonable for the subsequent ET. Furthermore, the fact that the ET from the 5-ASA anion to HO
2• was not observed in the CV results and the downhill HOMO–LUMO energies implies that the PT and ET occur concertedly, as the amino group with adjacent ring can donate both a proton and an electron.
The HOMO–LUMO relationship between the products after ET (i.e., the 5-ASA radical anion and HO
2−) is reversed, which is rational for orbital energies in the reverse ET (red dotted line in
Figure 6). However, the HOMO (−0.27731) of the PT-forming hydroperoxide (H
2O
2) is lower than the HOMO (−0.16694) of HO
2−, making the reverse ET impossible. Thus, the subsequent PT is dominant in determining the ET direction.
Analysis of the HOMO–LUMO relationship for O
2•− elimination by 5-ASA indicates that the concerted PCET involving two PTs and one ET occurs between the 5-ASA anion and O
2•− after initial deprotonation of the 1-carboxyl group. As reported previously, a concerted PCET occurs after the formation of the pre-reactive hydrogen-bonded complex from the free reactants, and the proton and electron are transferred in one kinetic step via a transition state forming the product complex [
11,
13]. Therefore, it is expected that the PCET between the 5-ASA anion and O
2•− occurs in a similar concerted mechanism through the HB formed between the amino group of the
p-AP moiety and O
2•−.
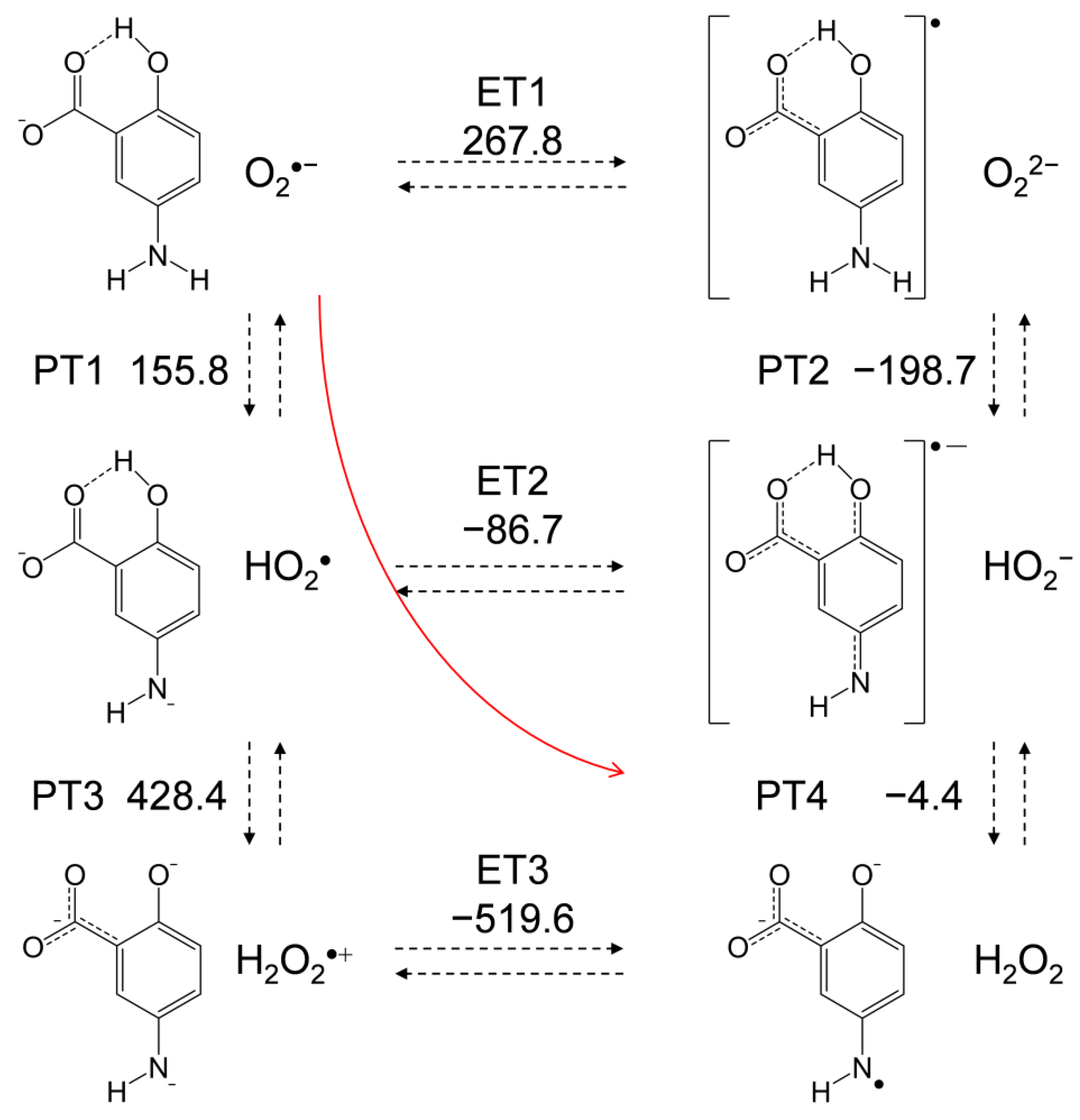
2.5. Free-Energy Analysis of PCET between Electrogenerated O2•− and the Deprotonated 5-ASA Anion
In
Figure 7, an equilibrium scheme and Δ
G° values for the six diabatic electronic states in the PCET between the deprotonated (−H
+) 5-ASA anion and O
2•− as calculated using DFT are shown. Since ET between HO
2• and the 5-ASA anion was not observed by CV, ET1 corresponding to oxidation of the 5-ASA anion by O
2•−, which has less oxidative power than HO
2•, is not feasible. Thus, PT1 must occur first, although both ET1 and PT1 are uphill endergonic reactions (Δ
G° = 267.8 and 155.8 kJ mol
˗1, respectively). In the following pathways, though PT3 is also endergonic, an exergonic ET2 (−86.7) followed by PT4 (−4.4) is a feasible pathway. Then, PT4 is expected to follow ET2 because of its exergonic Δ
G° (−4.4) (although the ESR spectrum observed for the reaction products could not unambiguously identify the terminal of the PCET pathway). In our previous study [
12], we determined that the second PT from the aminophenol moiety is necessary for the PCET, as is also demonstrated by the CV results (
Figure 2c), which revealed that
p-DAP, which has no amino proton, does not bring about successful HO
2• elimination. Additionally, analysis of the HOMO–LUMO relationship between 5-ASA and O
2•−, as shown in
Figure 6, indicates that the subsequent PT is the dominant factor that determines the ET direction. These findings imply that PT1 coupled with ET2–PT4 proceeds in a concerted manner without generating high energy intermediates, corresponding to the red line shown in
Figure 7.
For a comparative study, the Δ
G° values of the PCET pathways for
p-AP, the 4-ASA anion, and
m-AP were calculated (
Table 1). The total values of Δ
G° for the net PCET were obtained from the sum of the values for two PTs and one ET. From a thermodynamic viewpoint, the total values embody the energetic driving force of the net PCET for the 5-ASA anion and
p-AP. Conversely, the total value for the 4-ASA anion (214.4) cannot embody the energetic driving force because the Δ
G° for the unfeasible single ET has been summed in it. The important factor for the net PCET pathway comprised of PT1–ET2–PT4 to proceed as a sequential reaction is the Δ
G° values of the individual reactions. The acid−base interaction and the redox potentials of the components, rather than the total Δ
G°, are exergonic. In the pathway, the Δ
G° of PT4 (118.3) for the 4-ASA anion constitutes an uphill energy barrier to the net PCET reaction, and the same is true for that of ET2 (15.2) for
m-AP. Thus, these Δ
G° values confirm that PCET is feasible for the
p-AP moiety but not for the
m-AP moiety, supporting the other experimental results.
For the 5-ASA anion and
p-AP, PT1 is an uphill energy process, but the subsequent ET2 and PT4, are downhill. Therefore, a sequential pathway (PT1–ET2–PT4) is possible while the latter downhill reactions promote the net reaction equilibria. Alternatively, a concerted two-proton-coupled electron transfer (2PCET) may be a more feasible pathway than sequential PCET owing to its kinetic advantage (
Figure 7, red line). For the concerted pathway, PT1 will proceed with more efficient kinetics than single PT1 for sequential PCET involving one concerted kinetic step comprising ET2 and PT4. Furthermore, it should be noted that the subsequent reaction after the PCET equilibria is the irreversible decomposition of the product quinone radical via an intermediate amino radical, so the net PCET will be promoted.